Current and Future Treatments of EoE
The standard approach to EoE treatment consists of the “3 D’s:” diet, drugs, and dilation.1 Once EoE is diagnosed, shared decision making provides an excellent opportunity to discuss treatment modalities and patient preferences and values. Refer to the treatment algorithm in Figure 1.2,3

Diet. Elimination of food allergy triggers that promote an inflammatory response in the esophageal mucosa is the goal of dietary management strategies.4 There are three main dietary approaches to eliminating potential triggers4:
- Elemental diet. This is the most effective at bringing symptom remission, with a response rate of over 90%. This approach uses amino acid-based formulas (AAF) for intake, along with some treats. Elemental diets can interfere with development of feeding skills in babies, however, and a feeding tube may become necessary.
- Empiric dietary elimination. This diet follows the 6 Food Elimination Diet (SFED): dairy, wheat, eggs, soy, nuts and seafood. While less restrictive than the elemental diet, it only has a 60% to 70% response rate. Due to the restrictive nature of SFED, versions have been created that eliminate one (dairy), two (dairy and wheat), and four foods (dairy, wheat, egg, soy).
- Test-directed dietary modification: This diet eliminates food triggers identified skin prick testing (SPT) and/or atopy patch testing (ATP). It the least efficacious with response rates in approximately 40% of patients. Because of this low efficacy, IgE blood testing and SPT/APT no longer suffice to identify food triggers for patients with EoE.5 This test approach is also cumbersome, as patients must have metal disks in contact with skin for 48 hours.4
Patients treated with elimination diets should be referred to a registered dietitian if they experience excessive weight change, poor adherence, poor nutritional quality, and negative impact on quality of life. .1 Poor adherence to elimination diets, such as SFED, are influenced by effectiveness, social situations, and diet-related anxiety.6
Pharmacologic Therapy
There are two traditional choices of drug class: proton pump inhibitors (PPI) and oral formulations of topical corticosteroids.7 An initial trial of PPI therapy was standard of care until updated diagnostic guidelines in 2018. Now they are considered as one option for initial care.8 This approach is inexpensive and tolerable with a favorable safety profile.7
Swallowed topical corticosteroids have been routinely used in the treatment of EoE, with budesonide oral suspension receiving approval in 2024 for 12 weeks of treatment in those 11 years and older with EoE.9 A systematic review of double-blind clinical trials demonstrated that patients treated with topical corticosteroids for 2 to 12 weeks have approximately 3 times the rate of histological remission than placebo-treated patients (RR 0.39, 95% CI, 0.85 – 1.19). Topical steroids are well tolerated although Candida infections (12% to 15% of patients) can be a complication. There is no indication that adrenal insufficiency is a common side effect of topical steroid treatment (0.9%).10
Newer, targeted agents show promise for treating patients with EoE due to the type 2 (Th2) inflammation that drives its pathophysiology. In June of 2022, the FDA approved the first dupilumab for use in patients with EoE who are 12 years and older.11 Dupilumab has since received expanded approval in 2024, with approved indications for use in patients 1 year and older weighing at least 15kg.12 It binds to the interleukin (IL)-4 receptor α and suppresses the action of Th2 and type 2 innate lymphoid cells (ILC2) in response to IL-4 and IL-13 (Figure 2).7 Please see the animations using the menu for a more in-depth discussion of EoE pathophysiology and targeted agents.

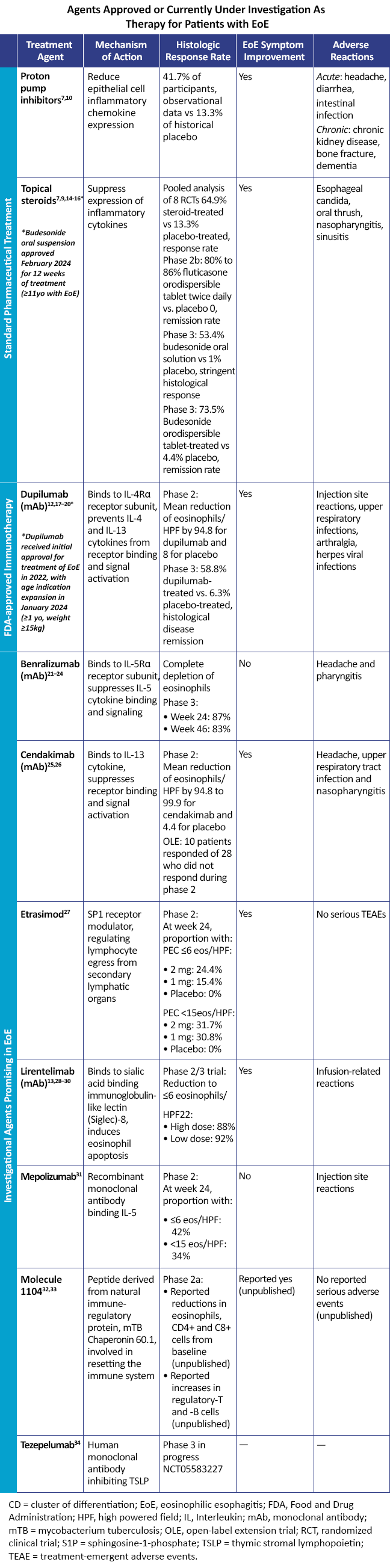
Numerous other agents are currently under investigation as therapy for patients with EoE.13 Table 1 compares the efficacy and safety of existing treatments to approved or investigational targeted therapies.
Dilation When EoE and inflammation has been ongoing for some time, esophageal strictures and narrowing may occur, leading to dysphagia and food impaction.7 Esophageal dilation can be successfully used to treat strictures but is normally used in patients after other treatments have failed. Studies do not support previous concerns regarding a high risk of complications, such perforation or ripping. 35 Patients most likely to benefit from dilation include: 35
- Those with strictures identified endoscopically with symptoms of dysphagia
- Those presenting with dysphagia symptoms in the absence of endoscopically apparent stricture
- Those who avoid symptoms of dysphagia because of adaptive eating behaviors and food avoidance, despite the presence of high-grade esophageal strictures (<10 mm)
References
- Shah A, Hirano I. Treatment of eosinophilic esophagitis: Drugs, diet, or dilation? Curr Gastroenterol Rep. 2007;9(3):181-8. doi:10.1007/s11894-007-0016-1
- Chen JW. Management of eosinophilic esophagitis: Dietary and nondietary approaches. Nutr Clin Pract. 2020;35(5):835-847. doi:10.1002/ncp.10571
- Sher ER, Ross JA, Weine DW, Arjun AC. Current and emerging therapies for eosinophilic esophagitis. Allergy Asthma Proc. 2022;43:178-186. doi:10.2500/aap.2022.43.220014 For access to this entire article and additional high-quality information, please check with your college/university library, local public library, or affiliated institution.
- Durban R, Dellon ES. Nutritional care of the patient with eosinophilic esophagitis. Practical Gastroenterol. 2018;17(4):40-51. Accessed July 25, 2022. https://practicalgastro.com/2019/07/30/nutritional-care-of-the-patient-with-eosinophilic-esophagitis
- Sampson HA, Aceves S, Bock SA et al. Food allergy: A practice parameter update-2014. J Allergy Clin Immunology. 2014;134:1016-25.e43. doi:10.1016/j.jaci.2014.05.013
- Wang R, Hirano I, Doerfler B, et al. Assessing adherence and barriers to long‑term elimination diet therapy in adults with eosinophilic esophagitis. Dig Dis Sci. 2018;63(7):1756-1762. doi:10.1007/s10620-018-5045-0
- Muir A, Falk GW. Eosinophilic esophagitis: A review. JAMA. 2021;326(13):1310-1318. doi:10.1001/jama.2021.14920
- Budesonide oral suspension (EohiliaTM) Prescribing Information (PI) 2024. (https://content.takeda.com/?contenttype=PI&product=EOH&language=ENG&country=USA&documentnumber=1). Accessed 5/15/24.
- Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: Proceedings of the AGREE conference. Gastroenterol. 2018;155(4):1022-1033.e10. doi:10.1053/j.gastro.2018.07.009
- Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterol. 2020;158(6):1776-1786.doi:10.1053/j.gastro.2020.02.038
- FDA approves first treatment for eosinophilic esophagitis, a chronic immune disorder. US Food & Drug Administration. Published May 20, 2022. Accessed July 25, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-eosinophilic-esophagitis-chronic-immune-disorder
- Dupilumab (Dupixent®) PI 2024 (https://www.regeneron.com/downloads/dupixent_fpi.pdf). Accessed 5/15/24.
- Khokhar D, Marella S, Idelman G, et al. Eosinophilic esophagitis: Immune mechanisms and therapeutic targets [published online ahead of print, 2022 Jul 1]. Clin Exp Allergy. 2022;10.1111/cea.14196. doi:10.1111/cea.14196
- Straumann A, Lucendo AJ, Miehlke S, et al. Budesonide orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic esophagitis. Gastroenterol. 2020;159(5):1672-1685. doi:10.1053/j.gastro.2020.07.039
- Dellon ES, Lucendo AJ, Schlag C, et al. Fluticasone propionate orally disintegrating tablet (APT-1011) for eosinophilic esophagitis: Randomized controlled trial. Clin Gastroenterol Hepatol. 2022;S1542-3565(22)00139-2. doi:10.1016/j.cgh.2022.02.013
- Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: Results from a phase 3 trial. Clin Gastroenterol Hepatol. 2022;20(3):525-534. doi:10.1016/j.cgh.2021.04.022
- Hirano I, Dellon ES, Hamilton JD, et al. efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterol. 2020;158(1):111-122.e10. doi:10.1053/j.gastro.2019.09.042
- Rothenburg M, Dellon E, Bredenoord A, et al. Dupilumab improves clinical and histologic aspects of disease in adult and adolescent patients with eosinophilic esophagitis at week 24: Results from part b of the 3-part LIBERTY EoE TREET study. J Allergy Clin Immunol. 2022;149(2):AB312. doi:10.1016/j.jaci.2021.12.003
- Collins MH, Lim WK, Wipperman MF, et al. Dupilumab improves histological outcomes and normalizes disease and type 2 inflammation transcriptome signatures in adult and adolescent patients with eosinophilic oesophagitis: Results from the 3-part phase 3 LIBERTY EoE TREET study. Abstract presented at: 2022 EAACI Annual Congress. Abstract 001140.
- Spergel BL, Ruffner MA, Godwin BC, et al. Improvement in eosinophilic esophagitis when using dupilumab for other indications or compassionate use. Ann Allergy Asthma Immunol. 2022 May;128(5):589-593. doi:10.1016/j.anai.2022.01.019
- Kuang FL, De Melo MS, Makiya M, et al. Benralizumab completely depletes gastrointestinal tissue eosinophils and improves symptoms in eosinophilic gastrointestinal disease. J Allergy Clin Immunol Pract. 2022;10(6):1598-1605.e2. doi:10.1016/j.jaip.2022.02.037
- NCT04534309. A study of benralizumab in patients with eosinophilic esophagitis (MESSINA). Updated June 3, 2022. Accessed August 8, 2022. https://clinicaltrials.gov/ct2/show/NCT04543409
- Fasenra (benralizumab). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
- NCT04543409. A study of benralizumab in patients with eosinophilic esophagitis (MESSINA); study results. 2023. https://classic.clinicaltrials.gov/ct2/show/results/NCT04543409. Accessed 5/15/24.
- Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterol. 2019;156(3):592-603.e10. doi:10.1053/j.gastro.2018.10.051
- Dellon ES, Collins MH, Rothenberg ME, et al. Long-term efficacy and tolerability of RPC4046 in an open-label extension trial of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2021;19(3):473-483. doi:10.1016/j.cgh.2020.03.036
- Dellon ES, et al. Efficacy and safety of the selective sphingosine 1-phosphate receptor modulator, etrasimod, in adult patients with eosinophilic esophagitis: primary results from the phase 2 VOYAGE study. Am J Gastroenterol. 2023;118(10S):S330-S331.
- Hirano I, Peterson KA, Murracy J, et al. AK002, an anti-siglec-8 antibody, depletes tissue eosinophils and improves dysphagia symptoms in patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2020;145(2):AB167. doi:10.1016/j.jaci.2019.12.343
- Dellon ES, Peterson KA, Murray JA, et al. Anti-siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med. 2020;383(17):1624-1634. doi:10.1056/NEJMoa2012047
- Dellon E, et al. Results from KRYPTOS, a phase 2/3 study of lirentelimab (AK002) in adults and adolescents with EoE. Am J Gastroenterol. 2022;117(10S):e316-e317. doi:10.14309/01.ajg.0000858424.48968.ad
- Dellon ES, et al. Mepolizumab for treatment of adolescents and adults with eosinophilic oesophagitis: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Gut. 2023;72:1828-1837.
- News release 4/19/23 (https://revolobio.com/2023/04/19/revolo-biotherapeutics-announces-positive-topline-data-from-phase-2a-trial-of-1104-in-adults-with-active-eosinophilic-esophagitis/). Accessed 5/15/24.
- News release 1/30/24 (https://revolobio.com/2024/01/30/revolo-biotherapeutics-receives-orphan-drug-designation-from-the-u-s-fda-for-its-first-in-class-peptide-as-a-potential-treatment-for-eosinophilic-esophagitis/). Accessed 5/15/24.
- NCT05583227. Efficacy and safety of tezepelumab in patients with eosinophilic esophagitis (CROSSING). https://www.clinicaltrials.gov/study/NCT05583227. Accessed 5/15/24.
- Katzka DA. Esophageal dilation as the primary treatment for eosinophilic esophagitis. Gastroenterol Hepatol. 2019;15(6):320-322.